In Vitro Diagnostic Medical Devices that are being sold in Europe or the UK are required to be CE Marked under the In Vitro Diagnostic Medical Devices Regulation (typically shortened to ‘IVD’).
There are a wide range of ‘devices’ that are intended to be used in medical applications and the majority of these ‘medical devices’ will come under the Medical Devices Regulation.
The Directive (EU 2017/746), which came into force on the 26th May 2022 applies to both devices and accessories, which could be a reagent, reagent product, calibrator, control material, kit, instrument, apparatus, equipment, or system, whether used alone or in combination of other devices.
In Vitro is Latin for in glass, which gives a good clue to as to what is in scope; effectively the In Vitro Diagnostic Medical Regulation (IVDMR) Directive applies to any medical device (as defined under the Medical Devices Regulation) which is intended to be used for the examination of specimens, including blood and tissue donations, derived from the human body, solely or principally for the purpose of providing information:
The procedure for CE marking is dependent upon the type of device and may need the involvement of a Notified Body; the types and procedures are summarised below:
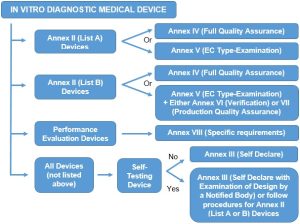
The conformity assessment for IVD Medical Devices often requires the involvement of a Notified Body, so therefore we are limited by the help that we can offer, however should you wish to talk to someone about CE marking and the conformity process, then please contact us as below: